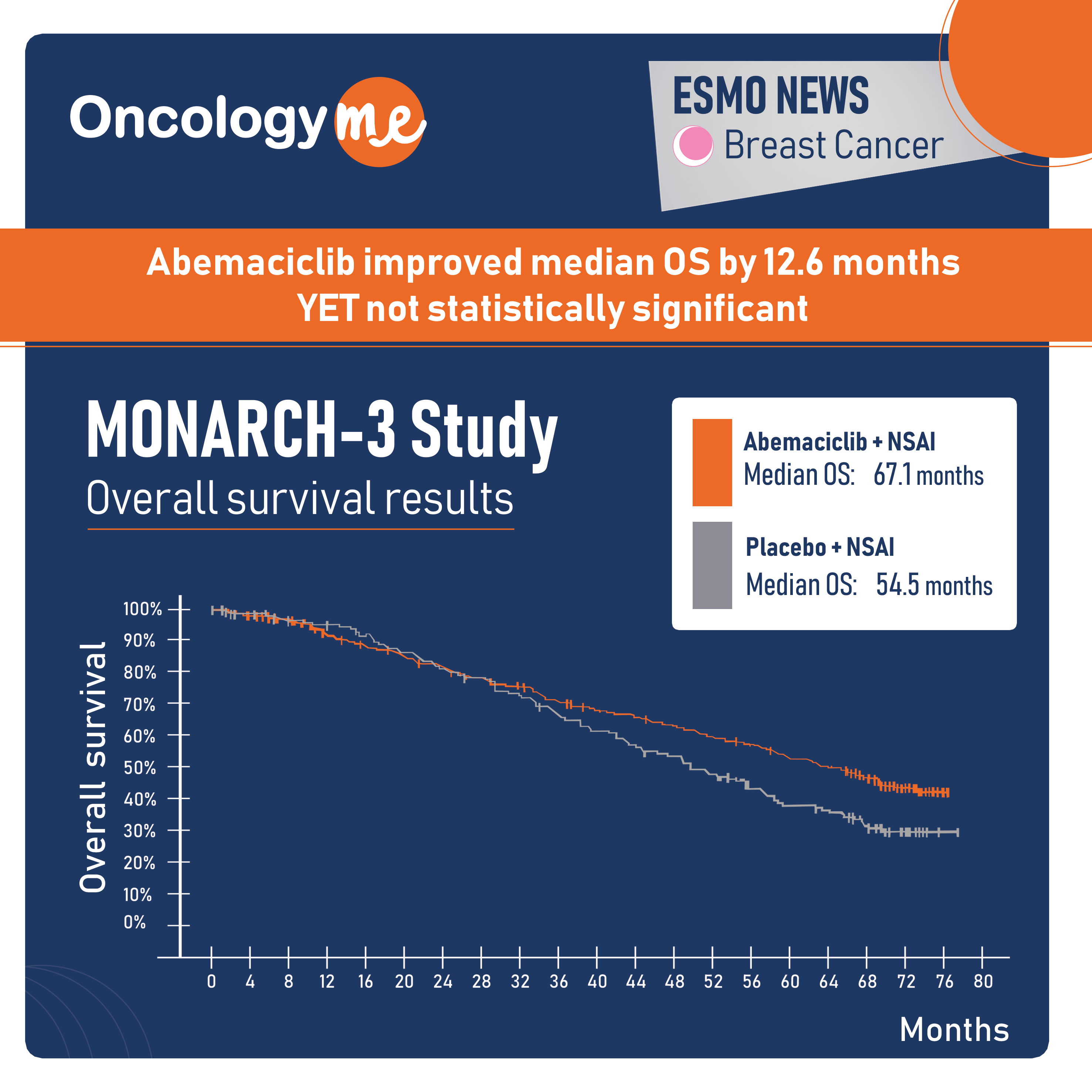
Monarch E Trial Results . Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; johnston srd, toi m, o'shaughnessy j, et al: 462 asian [22.8%], 54 black [2.7%],.
from www.oncologyme.com
Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. 462 asian [22.8%], 54 black [2.7%],. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; johnston srd, toi m, o'shaughnessy j, et al: In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65.
MONARCH3 trial For healthcare professionals only
Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. 462 asian [22.8%], 54 black [2.7%],. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; johnston srd, toi m, o'shaughnessy j, et al:
From www.youtube.com
Sara Hurvitz, MD, discusses the recently announced results of Monarch E Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: 462 asian [22.8%], 54 black [2.7%],. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; Monarch E Trial Results.
From www.researchgate.net
Relevant adverse events from phase III trials MONARCH2 and 3 [23,24 Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. 462 asian [22.8%], 54 black [2.7%],. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; Monarch E Trial Results.
From www.thelancet.com
Review of the monarchE trial suggests no evidence to support use of Monarch E Trial Results In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. johnston srd, toi m, o'shaughnessy j, et al: 462 asian [22.8%], 54 black [2.7%],. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; Monarch E Trial Results.
From www.semanticscholar.org
MONARCH 2 Abemaciclib in Combination With Fulvestrant in Women With Monarch E Trial Results 462 asian [22.8%], 54 black [2.7%],. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; johnston srd, toi m, o'shaughnessy j, et al: Monarch E Trial Results.
From www.edimark.fr
Essai Monarch E Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. 462 asian [22.8%], 54 black [2.7%],. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Monarch E Trial Results.
From www.researchgate.net
Main characteristics of study population and of MonarchE and Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. 462 asian [22.8%], 54 black [2.7%],. Monarch E Trial Results.
From www.studocu.com
Med Adverse Effects N180 Studocu Monarch E Trial Results 462 asian [22.8%], 54 black [2.7%],. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. johnston srd, toi m, o'shaughnessy j, et al: Monarch E Trial Results.
From www.oncologyme.com
MONARCH3 trial For healthcare professionals only Monarch E Trial Results Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; 462 asian [22.8%], 54 black [2.7%],. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. johnston srd, toi m, o'shaughnessy j, et al: Monarch E Trial Results.
From cevauhef.blob.core.windows.net
Monarch E Trial Jco at Joel Miller blog Monarch E Trial Results Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. 462 asian [22.8%], 54 black [2.7%],. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. johnston srd, toi m, o'shaughnessy j, et al: Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; Monarch E Trial Results.
From slideplayer.com
Moving Evidence into Practice Breast Cancer Highlights From Chicago Monarch E Trial Results In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; johnston srd, toi m, o'shaughnessy j, et al: 462 asian [22.8%], 54 black [2.7%],. Monarch E Trial Results.
From www.youtube.com
MONARCH 2 Overall survival of abemaciclib plus fulvestrant in HR+ Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: 462 asian [22.8%], 54 black [2.7%],. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Monarch E Trial Results.
From www.thelancet.com
Review of the monarchE trial suggests no evidence to support use of Monarch E Trial Results 462 asian [22.8%], 54 black [2.7%],. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; johnston srd, toi m, o'shaughnessy j, et al: Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Monarch E Trial Results.
From ascopubs.org
MONARCH 3 Abemaciclib As Initial Therapy for Advanced Breast Cancer Monarch E Trial Results Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; 462 asian [22.8%], 54 black [2.7%],. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. johnston srd, toi m, o'shaughnessy j, et al: Monarch E Trial Results.
From www.researchgate.net
Characteristics of patients fulfilling the monarchE inclusion criteria Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. 462 asian [22.8%], 54 black [2.7%],. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; Monarch E Trial Results.
From elmedicointeractivo.com
Monarch E consistencia durante 5 años El médico interactivo Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. 462 asian [22.8%], 54 black [2.7%],. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Monarch E Trial Results.
From vimeo.com
ESMO 2020 MONARCHE trial on Vimeo Monarch E Trial Results Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; 462 asian [22.8%], 54 black [2.7%],. Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. johnston srd, toi m, o'shaughnessy j, et al: In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Monarch E Trial Results.
From themedicalxchange.com
Gaining Further Perspective into Treatment of Advanced Breast Cancer Monarch E Trial Results johnston srd, toi m, o'shaughnessy j, et al: Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. 462 asian [22.8%], 54 black [2.7%],. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Monarch E Trial Results.
From www.wikidoc.org
Abemaciclib wikidoc Monarch E Trial Results Abemaciclib when combined with et is the first cdk4/6 inhibitor to demonstrate a significant improvement in. Of the 5637 patients (mean [sd] age, 49.9 [10.6] years; johnston srd, toi m, o'shaughnessy j, et al: 462 asian [22.8%], 54 black [2.7%],. In monarche (nct03155997), 4787 pts (84.9%) were aged <65 years and 850 pts (15.1%) were ≥65. Monarch E Trial Results.